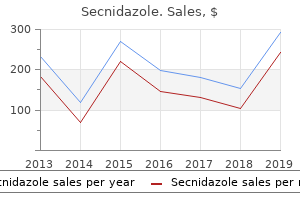
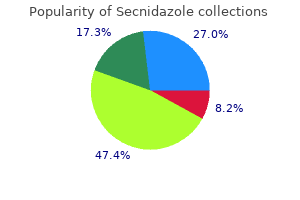
"Secnidazole 500mg amex, medicines360."
By: Lee A Fleisher, MD, FACC
- Robert Dunning Dripps Professor and Chair of Anesthesiology and Critical Care Medicine, Professor of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania

https://www.med.upenn.edu/apps/faculty/index.php/g319/p3006612
Pyridoxal phosphate participation in the transamination process is described in the following diagram: the aim of the exercise is to 20 medications that cause memory loss discount 500 mg secnidazole fast delivery demonstrate glutamate aminotransferase activity in the myocardium medications available in mexico discount secnidazole 500 mg otc. Incubate aspartate solution and -ketoglutarate solution with an extract of bovine heart muscle symptoms 3 dpo purchase secnidazole 500 mg without a prescription. As a result of glutamate aminotransferase, the present in the myocardium -ketoglutarate is converted to glutamate with simultaneous transformation of aspartate to oxaloacetate. Separated substances are applied to a spot approximately 3 cm from the bottom edge of the sheet (starting line) and then the paper is placed in the chromatography chamber. The paper is submerged in a few millimetres of a solvent mixture (mobile phase), so that the applied substances are above the surface of the solvent. Due to capillary action, the solvent moves gradually upward, dragging behind it at different speeds the ingredients present in the analyzed mixture. When the solvent front approaches the upper edge of the paper, the separation is complete. The individual components of the mixture follow the solvent front at different speeds. Due to the difference in migration velocities of the individual components of the mixture, at the end of the analysis they will be at different heights in relation to the starting line. When the separated compounds are colourful, these spots can be directly seen on the paper. If the components are colourless, they must be stained with the appropriate reagents. It is expressed as a ratio of the distance travelled by the component of the mixture (S1) to the distance travelled by the solvent front (S2). S1 Rf = S2 S1 the migration distance of a component S2 the migration distance of the solvent 124 the developed chromatogram shows the result of the process of transamination. After the incubation, filter both mixtures to separate test tubes and perform chromatography. At a distance of 3 cm from the bottom edge of the paper, draw a starting line with a pencil and mark four points at equal distances from one another (A, G, W, K). On these points, apply 3 drops of the appropriate solutions using a micropipette (apply a drop at a time, each time drying the paper): on point A 0. Chromatography should be continued until the solvent front is at a height of about 15 cm from the start line. Pay attention to the appearance of spots on the chromatogram with a Rf value corresponding to glutamate. Calculate the length of the polypeptide chain containing 120 amino acid residues, if: it occurs in the form of an -helix; it is fully extended. What is the total length of all polypeptide chains in the bacterial cell if it contains 106 molecules of protein and each of these molecules has a molecular weight of 40 kDa and is in the form: -helix, fully extended Calculate (in katals and international units) urease activity, which has turned a certain amount of urea into gaseous products with a total volume of 134. How many ml of gaseous products will be formed as a result of degradation (by urease) of urea, which was formed with the participation of 20 millimoles of aspartate
The reports of conditioned scurvy in humans are anecdotal and it does not represent a significant risk (Hornig and Moser symptoms 6 days before period due purchase secnidazole 500 mg with mastercard, 1981) medicine 93 3109 generic secnidazole 500mg fast delivery. Vitamin C increases iron uptake considerably from the gut when given to medicine wheel teachings discount 500mg secnidazole with amex humans in single-meal studies in amounts from 25 to 1000 mg (Hallberg, 1985; Cook and Monsen, 1977). Studies of longer duration show a less marked effect (Hunt and Roughead, 2000), but even a small increase could be important in subjects with conditions such as haemochromatosis (Gerster, 1999) or in subjects heterozygous for this condition. A dose of 2 g/day vitamin C taken with meals for 16 weeks in 17 healthy volunteers, and up to 24 months in 9 subjects, had no significant effect on body iron stores (Cook et al, 1984); this study was limited by the small numbers of participants and their variable iron status. Large amounts of vitamin C were reported to destroy the vitamin B12 content of food (Herbert and Jacob, 1974), and reduced vitamin B12 levels in serum were reported in 3 out of 90 individuals consuming more than 1000 mg/day of vitamin C over a minimum of 3 years (Hind, 1975). However, subsequent reports showed that these observations arose from inadequate assay methods (Newmark et al, 1976 and 1979), and that ascorbic acid in blood can interfere with the measurement of vitamin B12 (Herbert et al, 1978). An increase in serum cholesterol was reported in 25 patients with atherosclerosis following treatment with 1 g vitamin C daily for 6 weeks, but not in healthy volunteers (Spittle, 1971); the authors suggested that this may have arisen due to mobilisation of arterial cholesterol deposits (which would be a benefit), but there was no direct evidence to support this. There is conflicting evidence about the relationship between vitamin C intake and breast cancer. A prospective study in a large cohort (n=62,573) of postmenopausal women had found a lower risk of breast cancer in women with the highest intakes of vitamin C from food, but not from supplements (Verhoeven et al, 1997). However a recent nested case-control study found an increased risk of breast cancer among a cohort of postmenopausal Danish women (Nissen et al, 2003). A significantly increased risk was observed at intakes above 300 mg/day in comparison with intakes 60-150 mg/day. The numbers of cases and controls in the high-intake comparison were 62 and 41, respectively. When women who were taking supplemental vitamin C were excluded, the association between increasing vitamin C intake and breast cancer was weaker and no longer statistically significant. These have been performed at relatively low doses (280 mg/day, 60 or 260 mg/day and 500 mg/day for 6 weeks respectively), and there are no data currently available at higher intakes. Despite the extensive use of vitamin C supplements (up to 10 g/day) for the prevention of colds and other conditions, the tolerability of such intakes has not been subject to systematic assessment. Therefore there are few data to support the widely held view that high intakes of vitamin C are safe. There have been a small number of studies that have investigated dose-response relationships in a controlled and scientific manner. Gastrointestinal effects Two out of 15 volunteers experienced diarrhoea when consuming 10 g of vitamin C daily for 5 days (Wandzilak et al, 1994). Lower intakes appear to be tolerated without gastrointestinal effects since no subjective side effects were reported in 17 adults given 2 g/day for 16 weeks (Cook et al, 1984). The Miller and Hayes (1982) review concluded that doses greater than 1 g/day could result in adverse gastrointestinal effects. The data of Ludvigsson et al (1977) indicate that 1 g/day would not produce adverse gastrointestinal effects in children. Renal effects A review of the early investigational studies on the relationship between ascorbic acid intake and oxalate excretion (Hornig and Moser, 1981) concluded that there were methodological problems with many of the studies. A statistically significant increase in urinary oxalate excretion was reported in groups of 3 patients with calcium oxalate renal stones given 1 g or 2 g of supplemental ascorbic acid daily. The more extensive cross-over study by Wandzilak et al (1994) in which 15 subjects were given 1000, 5000 and 10,000 mg vitamin C each for 5 days reported no increase in oxalate excretion after correction for non-enzymatic ex vivo formation. However the data are difficult to interpret because of the highly acidic preservative used. The two studies by Auer et al (1998a and b) indicate no increase in the urinary excretion of oxalate in 10 healthy male volunteers (with no history of stone formation) given 4 g of vitamin C daily for 5 days, but a marked increase associated with haematuria and crystalluria in a single individual who took 8 g daily for a period of 8 days. In summary, one study reported that high intakes of vitamin C (1 or 2 g per day) increased the urinary excretion of oxalic acid in patients with renal stones, but this was not found in studies in healthy volunteers.
Secnidazole 500mg low cost. [3D AUDIO] SHINee - SYMPTOMS.

In 60 patients on long-term haemodialysis the relationship of serum Mo levels to treatment of bronchitis cheap secnidazole 500 mg online serum 2-microglobulin and C-parathyroid hormone levels and to schedule 6 medications purchase secnidazole 500mg visa the incidence of arthritis was investigated treatment definition math generic 500mg secnidazole with amex. Growth depression occurs in rats at 2-8 mg Mo/kg bw/day (Jeter and Davis, 1954; Miller et al, 1956) and skeletal changes at 7. In rabbits skeletal changes and nephrotoxicity were found at 5 mg Mo/kg bw/day (Asmangulyan, 1965), while skeletal changes, bodyweight loss and anaemia were seen at 25-46 mg Mo/kg bw/day (Arrington and Davis, 1953; McCarter et al, 1962). Adverse spermatogenic effects were seen in calves at 4 mg Mo/kg bw/day (Suttle and Field, 1969). Thiomolybdate intoxication can occur in experimental animals at intakes of 5 mg Mo/kg bw (Mills and Davis, 1987). From these studies the critical effects of molybdenum in the rat and mouse appear to be effects on reproduction, particularly foetal development. This study in rats is pivotal because of its satisfactory design, the use of an adequate number of test animals, demonstration of a clear dose-response relationship and clear toxicological endpoints. This comprises a factor of 10 for protecting sensitive human sub-populations with inadequate Cu intake or with deficient Cu metabolism in view of the species differences in antagonism between Mo and Cu, and another factor of 10 to cover the lack of knowledge about reproductive effects of Mo in humans and incomplete data on the toxicokinetics in man. Because the exposure in this 9-week rat study is sufficient to cover the relevant period of foetal development, a further uncertainty factor is unnecessary. Amino acid intolerance during prolonged parenteral nutrition reversed by molybdate therapy. The maximum permissible concentration of molybdenum in the water of surface basins. Mild renal failure induced by subchronic exposure to molybdenum, urinary kallikrein excretion as a marker of distal tubular effect. The effect of molybdenum levels in sorghum on uric acid and copper excretion in man. Federmann M, Morell B, Graetz G, Wyss M, Elsner P, von Thiessen R, Wuthrich B and Grob D (1994). The role of dietary molybdenum on oestrous activity, fertility, reproduction and molybdenum and copper enzyme activities of female rats. The effect of dietary molybdenum upon growth, hemoglobin, reproduction and lactation of rats. Changes of purine metabolism in man and animals under conditions of molybdenum biogeochemical provinces. Copper deficiency in cattle, sheep and horses caused by excess molybdenum from fly ash: case report. Inhibitory effects of molybdenum on oesophageal and forestomach carcinogenesis in rats. Demyelination in lambs born of ewes maintained on high intakes of sulphate and molybdate. Response of L-929 fibroblasts, human gingival fibroblasts, and human tissue mast cells to various metal cations. Induction of reverse mutation and mitotic gene conversion by some metal compounds in Sacch. Tests for carcinogenicity of metallic compounds by the pulmonary tumor response in strain A mice.
To incorporate these methods efectively into a coherent daily routine treatment lice order 500mg secnidazole otc, the staf of the blood establishment must be familiar with the ration ale behind the method symptoms xanax is prescribed for 500mg secnidazole sale, as well as its potential limitations and pitfalls treatment for sciatica order 500mg secnidazole fast delivery. This time can be reduced, for example, by placing plasma batches in a regular confguration to 168 Chapter 4 Principles of blood component processing maximise exposure to the freezing process. If a liquid environment is used, it should have been demonstrated that the container cannot be penetrated by the solvent (see Standards, Chapter 5, Component monographs, and the rele vant European Pharmacopoeia monographs for the required storage conditions of individual blood components for further fractionation and manufacturing of medicinal products derived from human plasma). Methods of thawing Frozen units should be handled with care since the bags may be brittle. The integrity of the pack should be verifed before and afer thawing to exclude any defects and leakages. Afer thawing of frozen plasma, the content should be inspected to ensure that no insoluble cryoprecipitate is visible. To preserve labile factors, plasma should be used as soon as possible afer thawing. Post-thaw shelf life may be extended for a validated period to facilitate urgent transfusion for some indications. Tawing of the plasma is an inevitable part of some current viral in activation processes, afer which the products may be refrozen within the production process. In order to preserve component quality, the fnal component should be used as soon as possible following thawing for clinical use and not further refrozen. In practice, this is done 169 Guide to the preparation, use and quality assurance of blood components by freezing units of plasma, thawing and then centrifuging them at low temperature. Details regarding the freezing, thawing, and centrifugation condi tions required for cryoprecipitate production are given in Standards, Chapter 5, Component monographs. Open and closed systems and sterile connection devices It is recommended that any new developments in component prepara tion involving an open system should be subjected to intensive testing during the developmental phase to ensure maintenance of sterility. Components prepared in systems using fully validated sterile connect ing devices may be stored as if prepared in a closed system. Monitor ing should be carried out by pressure testing of all connections and regular traction tests. Irradiation of cellular blood components Viable lymphocytes in blood components can cause fatal transfu sion-associated graf versus host disease, particularly in severely immune-compromised patients. Irradiation at doses specifed in the Standards does not cause signif icant harm to other blood cells. The risk of disease transmission is highest with fresh components containing mono and poly-morphonuclear leucocytes. Antibodies usually appear 4 to 8 weeks afer infection and can be demonstrated in standard screen ing tests. Since the infection is common, the test has to be repeated on each donation from a previously seronegative donor. Currently available systems have been demonstrated to inactivate a wide range of viruses, bacteria, parasites and leucocytes. Most clinical studies have demonstrated a reduced corrected count increment com pared to untreated control platelets, and one study found an increase in bleeding risk associated with this phenomenon. Other potential risks include toxicity and neo-antigen formation; neither has been observed in haemovigilance studies of short duration, but longer term surveillance studies will be required to confrm the absence of long-term toxicity. The value and cost-efectiveness of implementation of these tech nologies should be assessed in conjunction with current and alter native methods for risk reduction (see section 12, Bacterial safety of blood components). Purity of components Since blood components are used to correct a known defcit, each preparation process must be subjected to strict quality control. However, it is absolutely necessary to declare the quality and to be able to make diferent types of preparations in order to give the clinicians a reasonable choice for patients with diferent transfusion demands. For example, a red cell concentrate can be produced with varying concentrations of contaminating leucocytes and platelets. If the pro spective patient has antibodies against leucocyte antigens or if it can be foreseen that he/she will need a very large number of transfusions, leucocyte depletion will be more efective. Bacterial safety of blood components Overview Although blood collection and processing procedures are intended to produce non-infectious blood components, bacterial contamination may still occur.
References:
- https://pdfs.semanticscholar.org/8ded/a2384c03caa74095279f272bd7c82ff3007a.pdf
- https://www.paho.org/english/ad/fch/im/fieldguide_polio.pdf
- https://repository.ubn.ru.nl/bitstream/handle/2066/169320/169320.pdf?sequence=1
- http://d-scholarship.pitt.edu/18275/1/Genzlinger_2013_Thesis.pdf